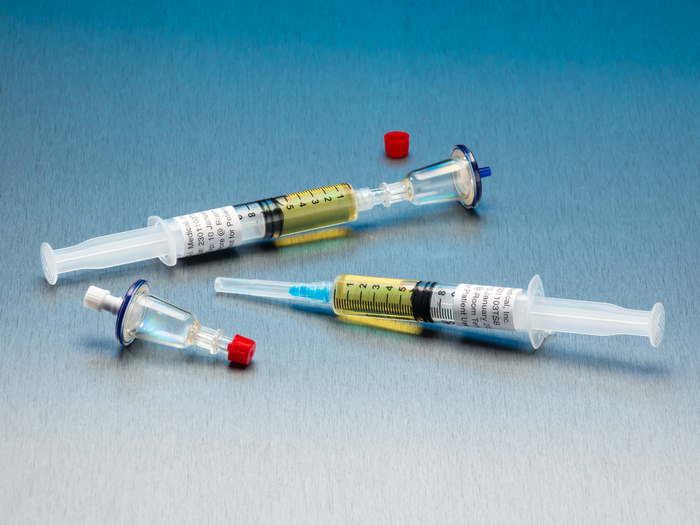
QT Micro™ System – TM6100
10 units / case
Overview
Full filtration microbial testing system for small volume compounded sterile products.
Non-destructive microbial test kit allows for sample to be used after testing.
FDA Registered Class 2 Device with required FDA Unique Device Identifier (UDI) barcode.
0.22µ contamination tester for syringe to sterile transfers.
For a kit with FTM syringes, see our QT Micro™ System – TM6101.
- 10 x QT Micro filter units with female luer lock inlet and male luer slip outlet
- 10 x 5cc syringes of TSB growth media
- Log sheet included
- Gummed labels included
Certificate of Analysis Search
Enter the Lot Number below for a PDF file of the COAs: